Elementary school students the world over are familiar with potato batteries or lemon batteries, but did you know you can make a battery from soda? With just some wire, two different kinds of metals, and your favorite soda, you can build your own battery!
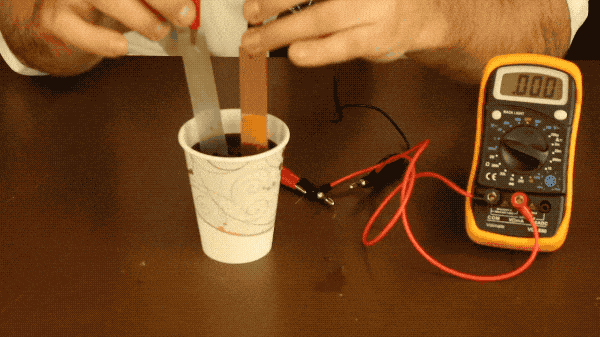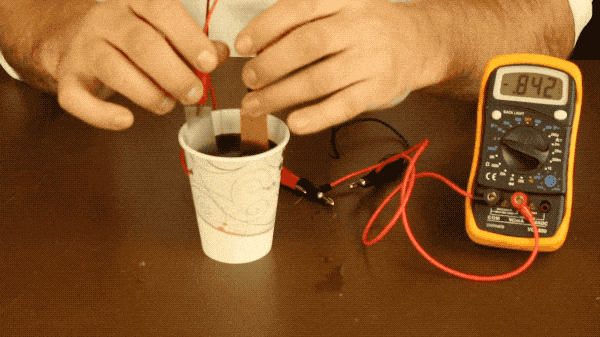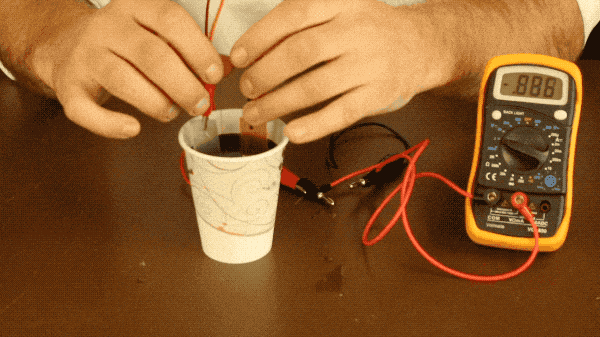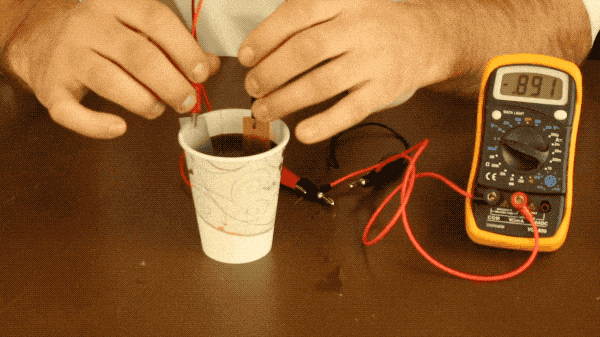
All you need to do is connect your metal plates to one another with a wire (or connect them to a multimeter as well to measure the battery’s voltage like we did) and place your plates into the soda. Make sure your plates aren’t touching during this experiment because if they touch, the electrons won’t flow through the wire and you won’t see a voltage register on the multimeter.
With our battery, the two metals we’re using are zinc and copper, as zinc is more reactive with the soda than the copper is; leading to a more significant voltage produced by the battery. You could also use the aluminum of the soda can, but you would need to remove the coating from the can with steel wool, as it is designed to prevent the aluminum and soda from reacting with one another. You need two different metals because if both plates are reacting with the soda the same amount, there’s no need for electrons to flow between them and therefor very little voltage produced like you see below when we try making a battery with two copper bars.
To understand how the voltage is generated, let’s take a look at the soda itself for a second. Within the soda is phosphoric acid; the key ingredient to this whole process. That phosphoric acid is breaking up into positive hydrogen ions and negative phosphate ions. Those phosphate ions are attracting the positive nuclei of the zinc and copper atoms, but copper holds its atoms together a little better than the zinc. As a result, a lot of electrons from the now separated zinc atoms are left over in the bar, some of which flow through the wire into the copper bar and establish an equilibrium.
The zinc bar is now fairly close to neutral, but the copper is more negative, so it attracts the positive hydrogen ions which remove some of the excess electrons and combine to form hydrogen gas. That gas floats up through the soda and out of the system. This process is happening in a continuous, rapid cycle so electrons are always flowing from the zinc to the copper.
Over time, the copper bar will start to become slightly positive and will repel some of its positive ions into the soda, some of which will encounter the excess electrons on the zinc bar and form a black coating of copper and copper oxide around the zinc. When that has gone on long enough, the zinc in the soda will be completely coated in copper and the voltage will look like our attempt with two copper plates above, eventually killing the battery.
When we tried with a different soda, Sprite, the voltage produced was roughly the same, but the battery lasted a lot longer because of the stronger acid in the Sprite. Grab your favorite soda and see how much voltage you can generate!



