All you need for this at-home science is a glass, a plate, a candle, water, a match, and a bit of caution, because we are dealing with fire.
Step 1: Pour the water on the plate
Step 2: Place the candle on the center of the plate

Step 3: Light the candle (or have a guardian light it for you)
Step 4: Place the glass down over the candle, step back and watch science happen!
This experiment is all about maintaining an equilibrium of air pressures inside and outside the glass. We usually experience air pressure as the force from the atmosphere pushing down on us. Here at AstroCamp we feel about twelve PSI, or pounds per square inch, of force which is the same amount of force that the water on the plate initially feels.
The flame heats up the air on the inside, creating a higher pressure than the air on the outside. Hotter air has a higher energy and therefore exerts more air pressure. The higher pressure pushes the water down and out of the glass. You should be able to see air bubbles in the water just outside of the glass from the air forcing the water out.
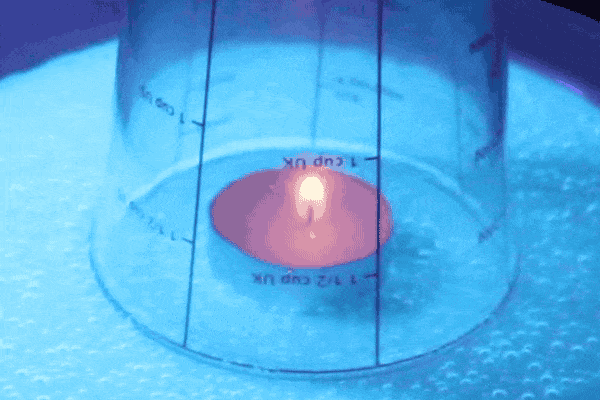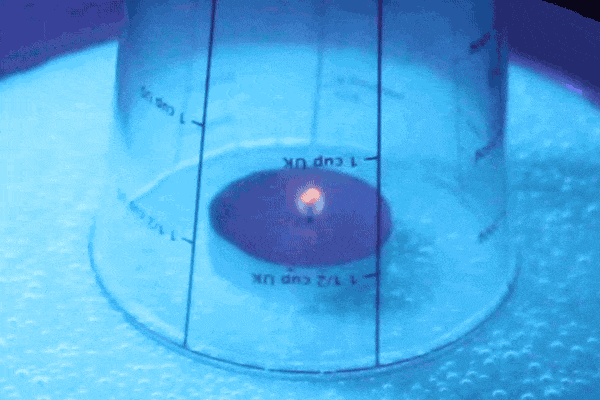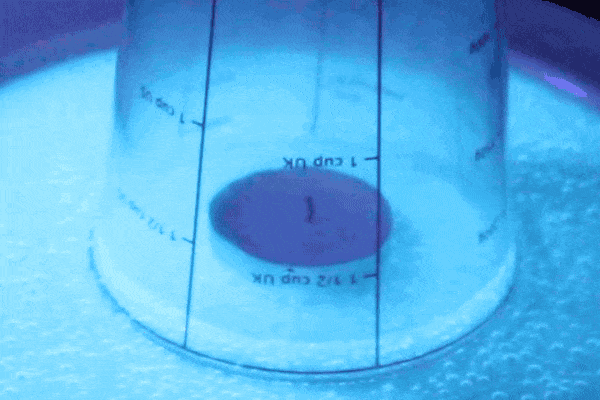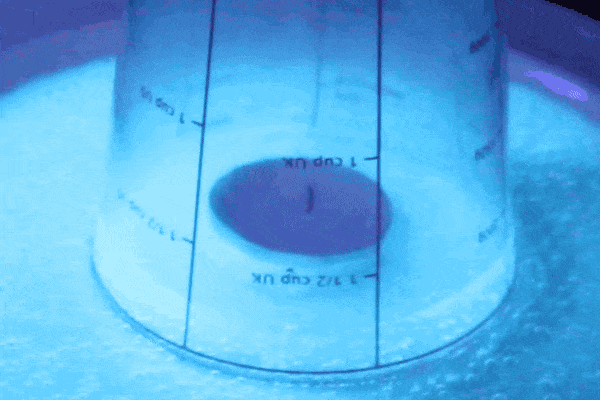
Fire needs oxygen in order to continue the combustion process in the glass, but because we trapped air in the there is only a finite amount. When the flame uses up all of the oxygen it goes out, which allows the air to cool. Cold air doesn’t have as much pressure as hot air and has less pressure than the air outside of the glass. Therefore the air on the outside of the glass pushes with greater force on the water than the air on the inside so the water is able to get sucked back up into the glass! When the water level evens out that is when you know you have reached an equilibrium of air pressures again.